Dissolution studies were first reported in 1897 (Noyes and Whitney) from which later the Noyes–Whitney equation was established. However, it was not until the 1950’s that the importance of dissolution on drug absorption rates was realized, and in the 1960’s its impact on the therapeutic effect. As a result, the dissolution test eventually became a quality control tool and compliance requirement with USP Apparatus 1 (basket-stirred test) introduced in 1971, Apparatus 2 (paddle method) in 1978, followed by a general USP chapter in 1985. Currently there are seven official USP apparatus described. The FDA released four dissolution guidances in 1997.
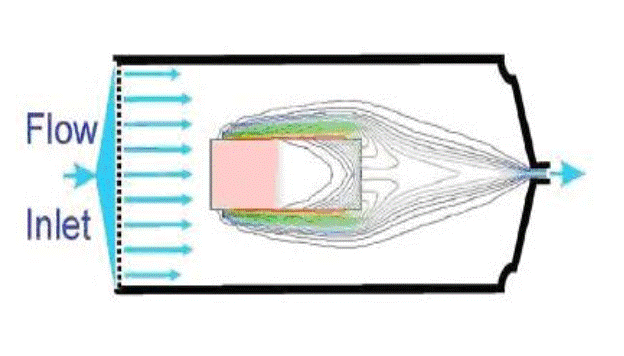
All dissolution measurements are impacted by convective-diffusion and hydrodynamics which are essentially uncontrolled in each of the currently prescribed apparatus. Flow immediate to the drug dissolution surface has been shown to have the greatest impact on the dissolution rate, having an impact of nearly the flow value (convection) squared, compared to a simple multiplication for the other factors of the Noyes-Whitney equation.
A body of work has been published and presented (Stevens, Missel, Weiner, et al) demonstrating the control of these parameters resulting in a highly reproducible and predictive high-performance dissolution system. This effectively represents a regulatory compliant modification of the USP Type 4 system producing dramatic improvements from the other manufactured systems on the market.
While there are additional methods and apparatus prescribed by the USP and other regulatory bodies, the following four represent the major types in current use. There are in addition, numerous variations of these and other specialized systems described for use. These, except for the previously modified Type 4 system described above rely on flows representing uncontrolled hydrodynamics and convection resulting complex and challenging results.
- USP Type 1 (basket-stirred apparatus )
- USP Type 2 (paddle apparatus)
- USP Type 3 (reciprocating cylinder apparatus)
- USP Type 4 (flow-through apparatus)
PharmaAnalytic has the demonstrated expertise to provide unique and comprehensive support to address the challenges associated with current dissolution systems and practices. In addition, PharmaAnalytic also represents a unique contribution in dissolution science developing high-performance dissolution systems capable of producing highly sensitive, relevant and predictive measurements with great precision and reproduciblity.